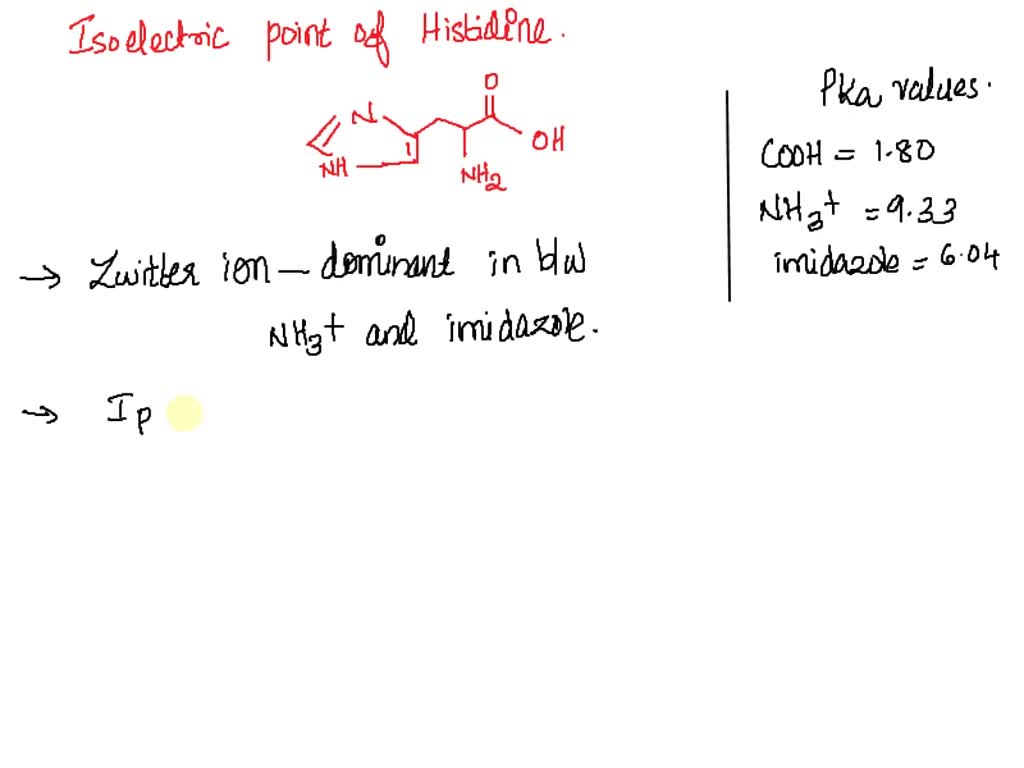
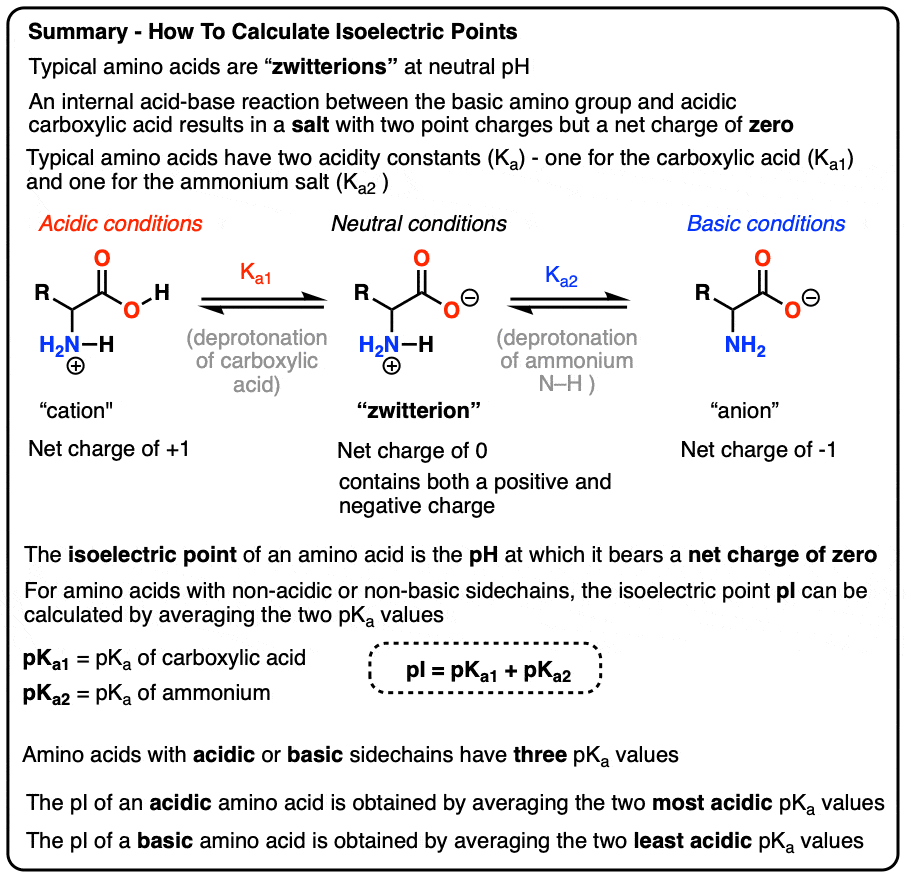
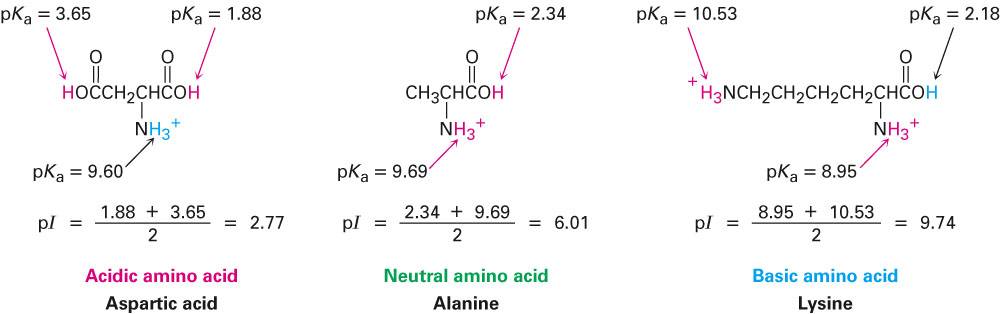
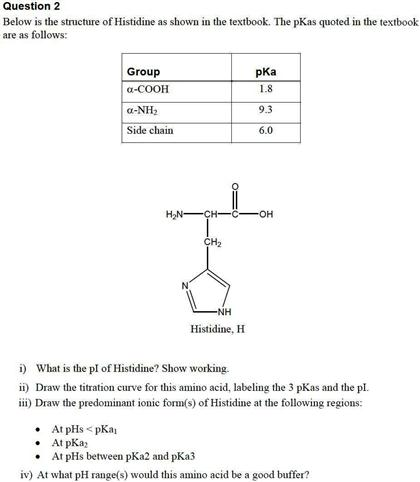
Define isoelectric point (pI)? How does it affect net charge on an amino acid? Calculate (show the calculations) the pI for histidine, and aspartic acid. pK values for these amino acids can
How is an isoelectric point calculated in amino acids containing three amino or carboxyl group? - Quora
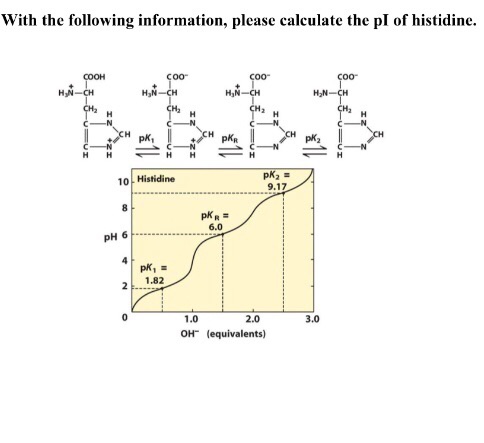
SOLVED: Using the values of pKas in the table below; calculate the pI for DTLH Properties of some amino acids found in proteins Abbreviations 1-and 3- pKa ofa-COOH Name pKa of a-NHy '
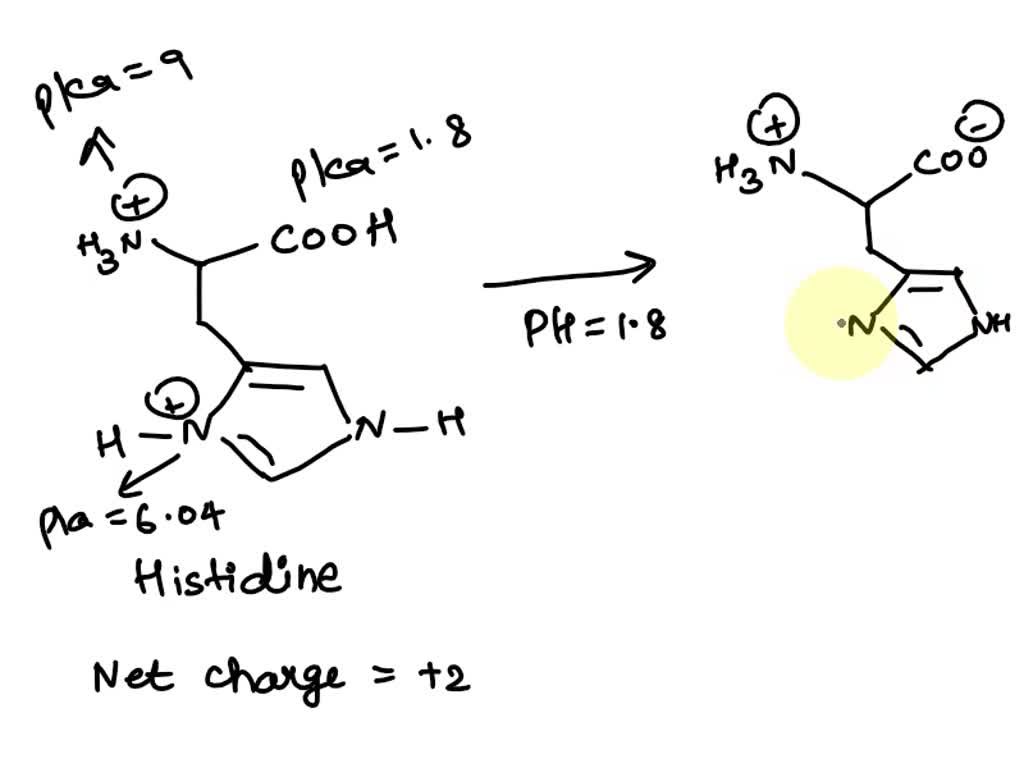
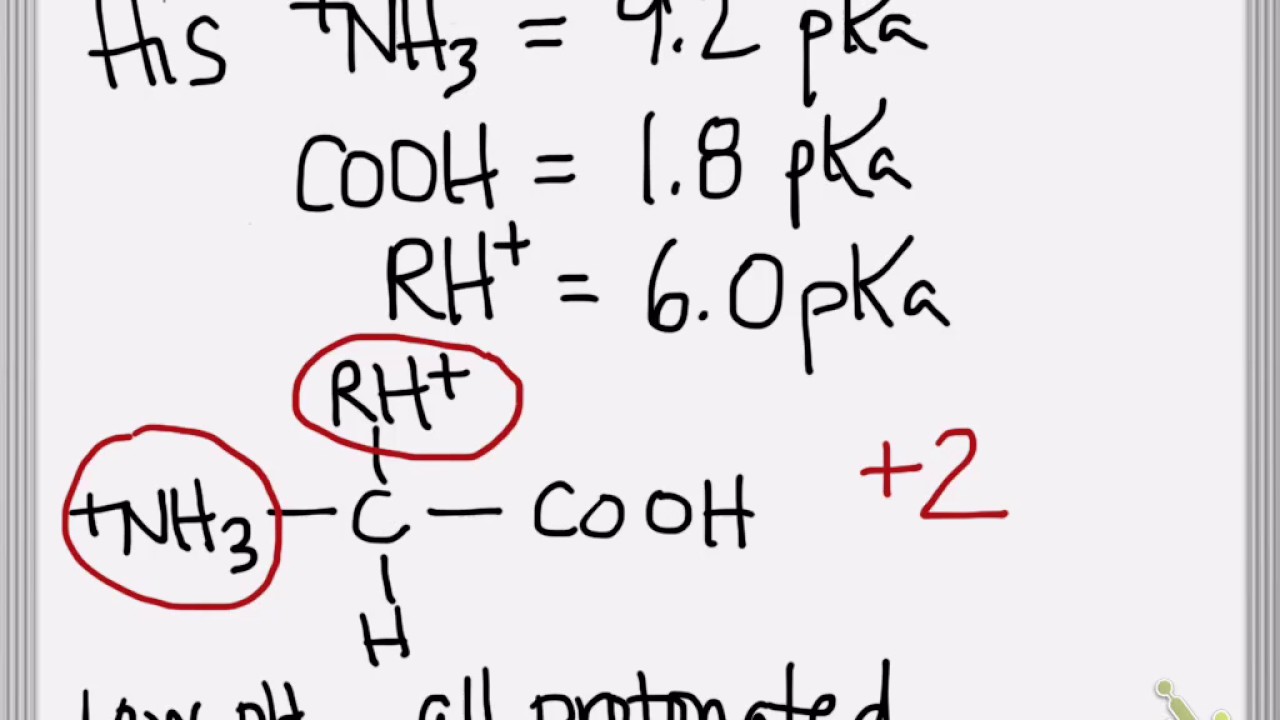
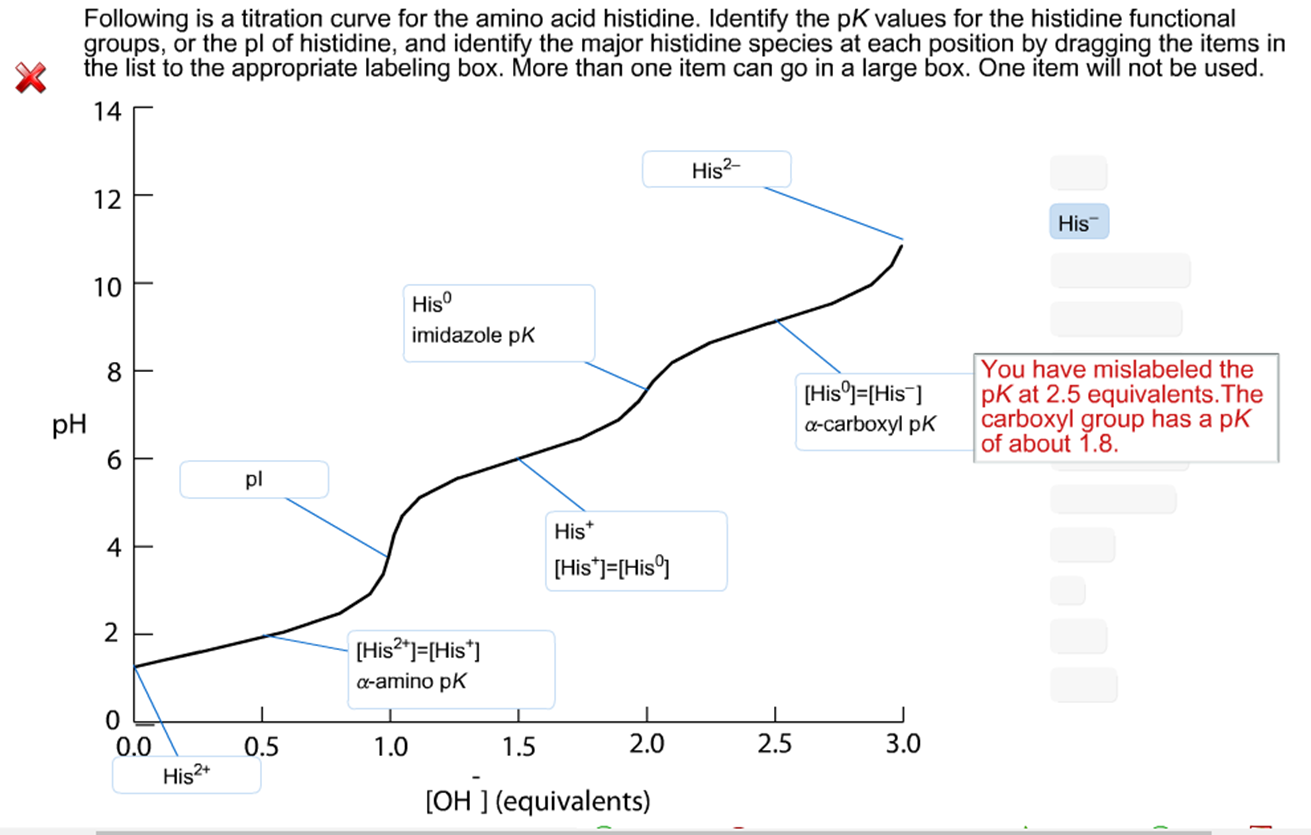
SOLVED: 1. Calculate the pI value of the histidine. Indicate the charge of the amino acid at each pKa value and show where the zwitterions are dominant

Draw a Titration Curve for each of the listed amino acids: a) Lysine,\\b) Cysteine, \\c) Glutamic Acid, \\d) Histidine, \\e) Tyrosine, \\f) Leucine. | Homework.Study.com
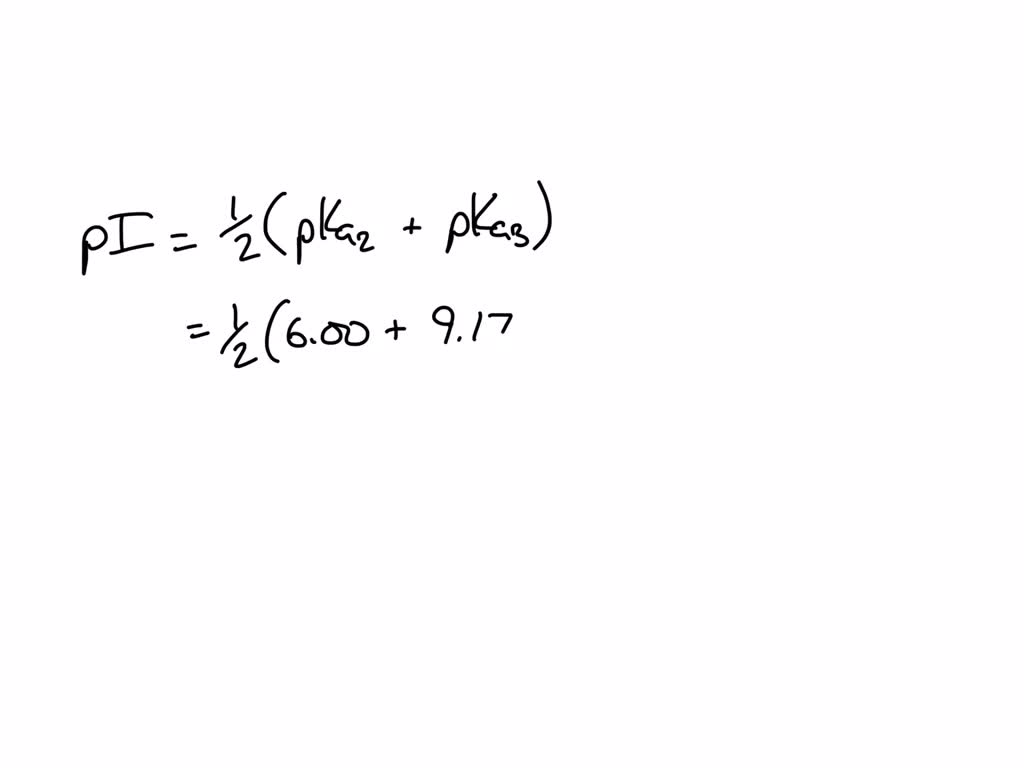
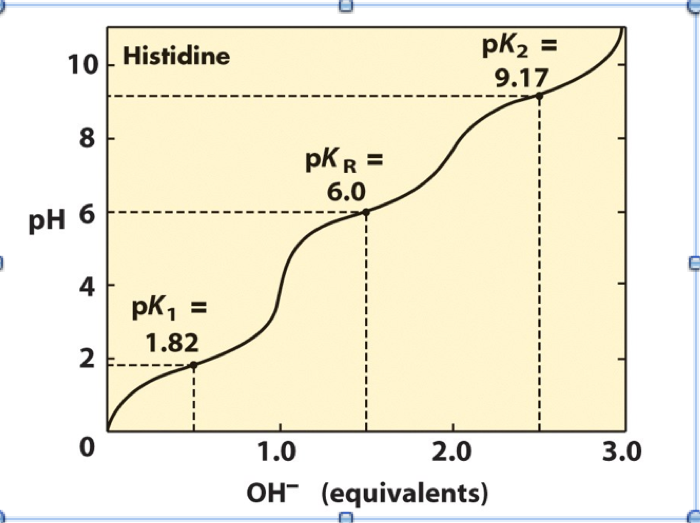
SOLVED: Given the pka values for the free amino acid, histidine (pk1 = 1.82, pKR = 6.00, pk2 = 9.17), calculate its isoelectric point. Options: 3.22 3.91 6.96 7.59